Location: Iran
Area: 9,000 sq.m
Project Cost: Confidential
Bio-containment level: BSL3+
Share:
PharmEng provided constructive review of the conceptual plans for cGMP compliance.
The client retained PharmEng Technology to apply knowledge and practical experiences with the World Health Organziation GMPs , and North Amercian US – FDA and Canada – Health Canada cGMPs to the planned facility in Iran.
PharmEng provided constructive review of the conceptual plans for cGMP compliance. This facility is complex in its design and processes as it will accommodate various functions at this site.
Responsibilities:
- review of the conceptual design for cGMP compliance including process layout, h functional relationships and space allocations

Exterior View (Conceptual)
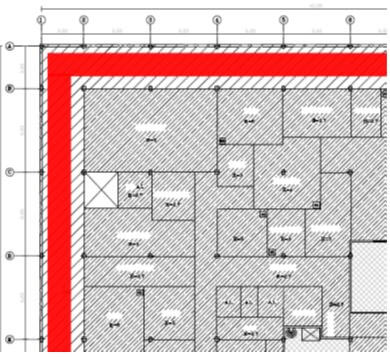
Layout Example
Location:
Iran
Area:
9,000 sq.m
Project Cost:
Confidential
Bio-containment level:
BSL3+
Share:






